
Exploring Stem Cell Peripheral Neuropathy: A New Era in Nerve Regeneration
Stem cell peripheral neuropathy treatment represents a revolutionary advancement in addressing chronic nerve damage caused by diabetes, chemotherapy, infections, or autoimmune diseases. In the first 60 words of understanding this treatment, we explore how regenerative medicine is opening doors for those who have suffered from numbness, tingling, burning, or weakness in their hands and feet. Mesenchymal stem cells (MSCs)—particularly those derived from Wharton’s jelly—are at the center of this promising therapy, providing anti-inflammatory, neuroprotective, and tissue-regenerating effects.
At Regenamex, patients with peripheral neuropathy now have access to non-surgical, safe, and effective solutions that target the root cause of nerve degeneration. These stem cells do more than suppress symptoms—they rebuild and revive damaged peripheral nerves. With increasing scientific support, stem cell peripheral neuropathy treatment is rapidly gaining attention as a preferred option for individuals looking to restore quality of life without relying solely on medications or invasive procedures.
How Stem Cell Therapy Targets Peripheral Neuropathy at the Cellular Level
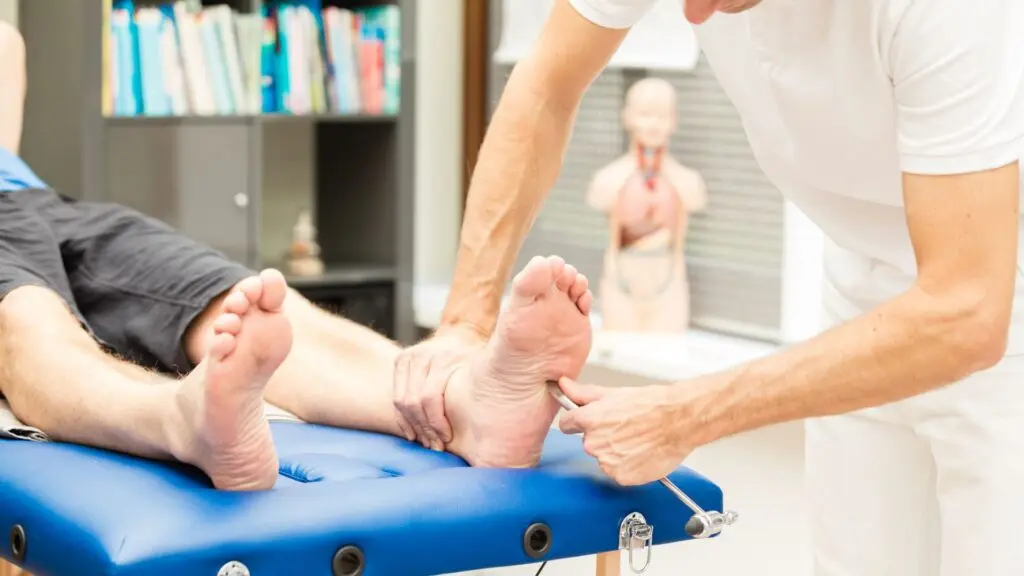
Stem cell peripheral neuropathy therapy works by introducing mesenchymal stem cells into the body, either through intravenous infusion or direct injection near affected nerves. These MSCs are attracted to sites of inflammation and degeneration, where they release bioactive molecules that support healing. Among their functions: reducing inflammation, repairing myelin sheaths around nerves, promoting angiogenesis (new blood vessel growth), and modulating the immune system to prevent further damage.
Wharton’s jelly-derived MSCs, in particular, are ideal for this process because they are young, potent, and contain a high concentration of cytokines and growth factors. As these cells settle in, they begin creating a supportive microenvironment around damaged nerve tissue. Over time, this can lead to functional improvements, decreased pain and numbness, and better mobility. For patients struggling with diabetic neuropathy or chemotherapy-induced nerve damage, this cellular approach offers more than temporary relief—it promotes true regeneration.
Who Is a Good Candidate for Stem Cell Therapy for Neuropathy?
Many people suffering from chronic peripheral neuropathy may be unaware that they are eligible for regenerative stem cell treatment. This therapy is ideal for patients who have tried traditional options—such as antidepressants, anticonvulsants, or physical therapy—without significant results. It’s also suited for those who want to avoid the long-term side effects of pharmaceutical interventions.
The most common conditions that benefit from stem cell peripheral neuropathy treatment include:
-
Diabetic neuropathy
-
Chemotherapy-induced peripheral neuropathy (CIPN)
-
Autoimmune-related nerve damage (e.g., lupus, MS)
-
Post-infectious or viral neuropathies
-
Chronic nerve pain from spinal injury or surgery
Eligible candidates are typically adults aged 30–80 experiencing persistent symptoms like numbness, weakness, burning sensations, or tingling in their extremities. Patients with underlying inflammatory conditions or who have failed to respond to conventional treatments are also strong candidates for this regenerative approach.
What Kind of Results Can Patients Expect From Treatment?

Understanding the realistic outcomes of stem cell peripheral neuropathy treatment helps set proper expectations. The process of healing nerves takes time, but patients typically notice early signs of improvement such as reduced burning, tingling, or pain within the first four weeks. As the stem cells begin their repair work, other changes follow in mobility, balance, and endurance.
Here’s a general recovery timeline:
-
Weeks 1–4: Reduced inflammation, less pain or tingling
-
Weeks 4–8: Improved foot/hand sensation, better gait and movement
-
Months 3–6: Nerve repair progresses, enhanced reflexes, greater independence
-
Beyond 6 months: Stabilized results, long-term nerve recovery, and higher quality of life
Patients who combine treatment with healthy lifestyle habits—like blood sugar control, hydration, and physical therapy—often achieve the most significant and long-lasting improvements. At Regenamex, every protocol is personalized to the patient’s condition, ensuring optimal results for each case.
Why Regenamex Uses Wharton’s Jelly MSCs for Neuropathy Relief
Not all stem cells are created equal, and the source matters immensely when it comes to successful peripheral neuropathy treatment. Regenamex uses only mesenchymal stem cells derived from Wharton’s jelly in the umbilical cord. These cells are harvested ethically from full-term donations and are biologically younger and more potent than those taken from fat or bone marrow.
Wharton’s jelly MSCs offer several key advantages:
-
Non-invasive collection process (no surgery required)
-
Superior anti-inflammatory and neuroprotective properties
-
Increased cell viability and regenerative power
-
Low risk of immune rejection or inflammation
Compared to adult-derived MSCs, Wharton’s jelly cells contain higher concentrations of healing molecules and haven’t been compromised by age, illness, or metabolic disorders. This gives them a distinct edge in repairing sensitive nerve tissue. Regenamex’s decision to avoid fat and bone marrow stem cells reflects a commitment to the highest safety and efficacy standards.
How Is Stem Cell Peripheral Neuropathy Treatment Administered?

At Regenamex, stem cell therapy is delivered with precision, safety, and patient comfort in mind. After thorough diagnostic review, patients receive customized protocols based on their type and severity of neuropathy. The typical dosage ranges from 1 to 5 million mesenchymal stem cells per kilogram of body weight, with most patients receiving one to three sessions over several days.
Delivery methods include:
-
Intravenous infusion for widespread nerve symptoms
-
Localized injection for targeted treatment near damaged nerves
The procedure is performed in a licensed regenerative facility with sterile conditions and ultrasound or fluoroscopic imaging for guidance. Patients typically remain in Mexico for 2–5 days, with support for travel, lodging, and translation provided by our team. After treatment, lifestyle and mobility guidelines are shared to help maximize stem cell performance and integration.
Why International Patients Trust Regenamex for Neuropathy Stem Cell Therapy
Regenamex is more than a regenerative medicine clinic—it’s a trusted resource for patients worldwide seeking cutting-edge solutions for chronic health issues. Located in Mexico and fully licensed under national health regulations, Regenamex has become a go-to destination for individuals seeking stem cell peripheral neuropathy treatment with ethical standards and proven protocols.
Our team provides:
-
Board-certified doctors trained in regenerative science
-
Exclusive use of Wharton’s jelly MSCs (no adipose or bone marrow)
-
Transparent pricing from $2,500 to $12,000 USD
-
Concierge support for international patients, including airport pickup
-
Personalized treatment plans with post-care follow-up
Explore our Stem Cell Therapy Mexico page for full details or visit the Contact Page to schedule your consultation. For additional research, read this PubMed study on MSCs in neuropathy or the Mayo Clinic’s resource on stem cell therapy.
Frequently Asked Questions About Stem Cell Peripheral Neuropathy
Stem cell peripheral neuropathy refers to the application of regenerative medicine—specifically mesenchymal stem cells (MSCs)—to treat damage in the peripheral nervous system. These nerves, which lie outside the brain and spinal cord, are often compromised by diabetes, chemotherapy, infections, or autoimmune conditions. The treatment involves introducing MSCs into the body through intravenous or localized injections. Once delivered, these powerful cells migrate to areas of damage where they release growth factors, cytokines, and neuroprotective molecules that help calm inflammation, repair myelin sheaths, stimulate new nerve fiber growth, and restore healthier nerve signaling. Over time, this biological process can reverse the underlying nerve dysfunction rather than simply masking symptoms, leading to restored sensation, improved mobility, and better quality of life.
Every patient’s healing timeline is different, but nerve regeneration is inherently a gradual process. Some patients may begin to notice subtle improvements—such as less tingling or a slight return in sensation—within a few weeks after their stem cell treatment. However, more significant changes often become apparent between the second and fourth month. As the stem cells continue repairing and rebuilding nerve tissue, improvements compound. Most patients see their peak results between six and twelve months post-treatment, especially when following a comprehensive post-care plan that includes physical activity, nutritional support, and medical follow-ups.
Stem cell peripheral neuropathy therapy can be highly effective across several types of nerve damage. Diabetic neuropathy is among the most common and most responsive, as the underlying inflammation and microvascular damage can be addressed directly by the anti-inflammatory and regenerative capabilities of MSCs. Chemotherapy-induced peripheral neuropathy (CIPN) also responds well, as stem cells can help repair oxidative stress and neurotoxicity from previous cancer treatments. Autoimmune-related neuropathies, such as those associated with lupus or multiple sclerosis, benefit from MSCs’ ability to modulate immune activity and reduce ongoing damage. Even post-infectious and injury-related nerve damage can show meaningful improvement with regenerative therapy.
Patients often worry about discomfort, but stem cell peripheral neuropathy treatment is considered minimally invasive and well tolerated. The most common delivery method—intravenous infusion—is similar to receiving a vitamin IV and usually causes no discomfort. Localized injections near nerve clusters may involve a brief sting but are done under precise image guidance for accuracy and safety. Post-treatment, some patients may feel mild soreness, fatigue, or a sensation of internal "healing," but these side effects are typically short-lived. Most people are able to resume light activity the same or next day without the need for downtime or heavy medication.
The choice of stem cell source is one of the most critical factors in treatment effectiveness. Wharton’s jelly MSCs are harvested from umbilical cord tissue after healthy, full-term births—no surgery, no harm to mother or baby. These cells are younger and more biologically active than stem cells taken from adult fat or bone marrow, which often lose potency with age and environmental exposure. Wharton’s jelly MSCs contain a higher density of growth factors, cytokines, and healing molecules, making them ideal for sensitive nerve tissue repair. Additionally, their low immunogenic profile means they are far less likely to trigger an immune response, increasing safety and compatibility.
Regenamex offers transparent pricing based on the scope and customization of each patient’s treatment plan. The typical cost ranges from $2,500 to $12,000 USD and depends on variables like the number of MSCs administered, the type of delivery method (IV vs. localized), and whether multiple sessions are required. Unlike many clinics, Regenamex provides clear cost outlines and comprehensive support to help patients make informed decisions. During your consultation, you'll receive a personalized recommendation and pricing structure based on your condition and medical goals.

