
Stem Cell Injection for Back
Stem cell injection for back pain is gaining global attention as a non-surgical, regenerative option for individuals suffering from chronic spinal conditions that fail to respond to conventional treatments. Back pain is one of the leading causes of disability worldwide, often driven by disc degeneration, chronic inflammation, nerve compression, or deterioration of spinal joints. Over time, these conditions can significantly impact mobility, sleep quality, work performance, and overall quality of life. Instead of masking symptoms with painkillers or temporary injections, stem cell injection for back pain focuses on improving the biological environment within the spine that contributes to pain, stiffness, and functional decline.
Unlike surgery or long-term medication use, stem cell injection for back conditions aims to reduce inflammation, support tissue health, and restore healthier cellular communication within damaged spinal structures. At Regenamex, this regenerative approach is delivered using advanced mesenchymal stem cells (MSCs) derived exclusively from ethically donated placental tissue and Wharton’s jelly. These biologically young and potent cells allow patients to access cutting-edge regenerative care in a regulated medical setting, offering a forward-thinking option for individuals seeking relief without invasive procedures.
Understanding Chronic Back Pain and Degenerative Spine Conditions
Chronic back pain rarely originates from a single injury or isolated event. In most cases, it develops gradually as spinal discs lose hydration, facet joints become arthritic, and surrounding ligaments and muscles weaken or stiffen. Factors such as aging, repetitive physical stress, poor posture, obesity, prior trauma, and inflammatory conditions all accelerate spinal degeneration. As discs lose their cushioning ability, abnormal movement and nerve irritation can occur, leading to persistent pain, stiffness, radiating symptoms, and reduced flexibility.
Traditional treatment pathways often focus on managing symptoms rather than correcting the underlying biological dysfunction. Physical therapy, oral medications, and steroid injections may temporarily reduce discomfort, but they do not repair damaged tissue or slow degenerative progression. When conservative measures fail, patients are frequently advised to consider surgery, which may involve disc removal, fusion, or hardware placement. Stem cell injection for back pain offers an alternative approach—one that targets inflammation, cellular dysfunction, and tissue breakdown before structural damage becomes irreversible.
How Stem Cell Injection for Back Pain Works
Stem cell injection for back pain does not involve surgical incisions, implants, or permanent alterations to spinal anatomy. Instead, stem cell therapy for back pain relies on the powerful biological signaling abilities of mesenchymal stem cells. Once administered—either through targeted spinal injections or intravenous infusion—MSCs naturally migrate toward areas of inflammation and tissue damage within the spine, guided by chemical signals release.
Rather than transforming directly into disc or bone cells, MSCs act as regulators of the healing environment. They release growth factors, cytokines, and extracellular vesicles that help calm excessive inflammation, regulate immune responses, and promote healthier cellular activity. This signaling supports disc hydration, protects nerve structures, and improves communication between spinal tissues. By influencing multiple biological pathways simultaneously, stem cell injection for back pain provides a more comprehensive approach than treatments that target only pain perception.
Mesenchymal Stem Cells for Back Pain Relief
Mesenchymal stem cells play a central role in regenerative spine care due to their versatility, safety profile, and strong anti-inflammatory properties. Unlike embryonic stem cells, MSCs pose no ethical concerns and have been widely studied for their immunomodulatory and tissue-supporting effects. Their ability to influence healing without provoking immune rejection makes them especially valuable for treating chronic spinal conditions.
Regenamex exclusively uses placental-derived MSCs and Wharton’s jelly stem cells, which are biologically younger and more potent than adult-derived stem cells. These cells exhibit enhanced regenerative signaling, stronger anti-inflammatory effects, and greater cellular communication capabilities. Because chronic back pain is often driven by ongoing inflammation rather than acute injury, these qualities are particularly beneficial in helping stabilize spinal health and slow degenerative processes.
Stem Cell Injection for Back vs Conventional Spine Treatments
Understanding how regenerative therapy compares to conventional spine treatments helps patients make informed decisions about their care. Traditional approaches such as medications, steroid injections, or surgery often focus on symptom suppression or mechanical correction. While these options may provide short-term relief, they rarely address the biological environment responsible for degeneration.
Stem cell injection for back pain offers a fundamentally different strategy—one centered on preserving existing tissue, reducing inflammation, and supporting long-term spinal health. Rather than removing or replacing damaged structures, regenerative therapy aims to optimize the spine’s internal repair mechanisms, potentially delaying or avoiding invasive procedures.
| Aspect | Conventional Back Treatment | Stem Cell Injection for Back |
|---|---|---|
| Primary Focus | Symptom management | Cellular repair & modulation |
| Effect on Degeneration | Does not reverse damage | May slow or stabilize progression |
| Invasiveness | Often surgical | Non-surgical |
| Recovery Time | Weeks to months | Minimal downtime |
| Long-Term Strategy | Lifelong medications or surgery | Regenerative support |
Conditions Commonly Treated With Stem Cell Therapy for Back Pain
Stem cell therapy for back pain may benefit patients with a wide range of degenerative and inflammatory spine conditions. It is particularly well-suited for individuals whose symptoms persist despite conservative care but who are not ideal candidates for surgery. Conditions driven by chronic inflammation and tissue breakdown tend to respond best to regenerative approaches.
Commonly treated conditions include degenerative disc disease, herniated or bulging discs, facet joint arthritis, chronic lower back pain, sciatica, nerve irritation, and post-surgical back pain. At Regenamex, every patient undergoes a comprehensive evaluation to determine whether stem cell injection for back conditions is appropriate and how it should be integrated safely into an individualized treatment plan.
Clinical Research Supporting Regenerative Treatment for Spine Health
Scientific interest in regenerative spine therapies has grown rapidly over the past decade. Preclinical research and early-stage clinical studies suggest that mesenchymal stem cells may reduce inflammatory markers, improve disc hydration, and support healthier spinal biomechanics. These biological effects are closely linked to improvements in pain levels, mobility, and daily function.
Although large-scale randomized trials are still ongoing, real-world clinical data continues to show encouraging outcomes for selected patients. Many individuals receiving stem cell injection for back pain report reduced pain intensity, improved flexibility, fewer flare-ups, and enhanced quality of life. Regenamex continuously integrates evolving research into evidence-based protocols that emphasize safety, transparency, and realistic expectations.
The Regenamex Approach to Stem Cell Injection for Back Conditions
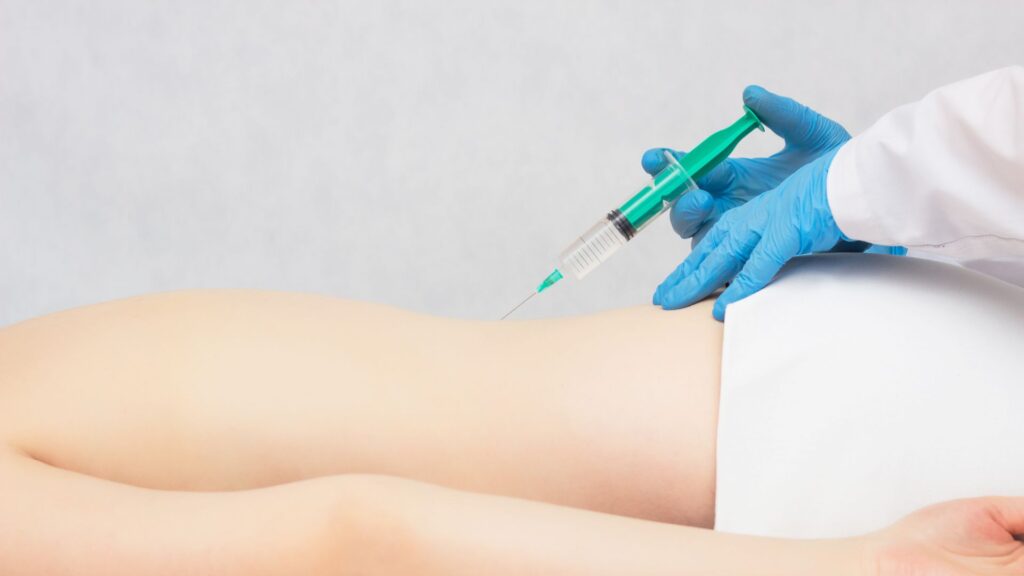
Regenamex delivers stem cell injection for back pain under strict medical and regulatory standards, prioritizing patient safety and individualized care. Each treatment plan is designed based on medical history, imaging studies, symptom severity, and functional goals.
Regenamex protocols include ethically sourced placental and Wharton’s jelly MSCs, COFEPRIS-regulated laboratory processing, sterility and potency testing, physician-guided administration, and personalized dosing strategies. Complementary therapies such as PRP, exosomes, IV nutrition, and hormone optimization may also be incorporated to enhance regenerative outcomes and support long-term spine health.
Safety and Regulation of Stem Cell Therapy in Mexico
Regenerative medicine in Mexico is regulated by COFEPRIS, the federal authority overseeing advanced medical therapies. Regenamex operates fully within this regulatory framework, ensuring ethical sourcing, laboratory compliance, and physician oversight at every stage of care.
Mesenchymal stem cells used for back pain treatment have demonstrated an excellent safety profile. Most patients experience only mild, temporary effects such as soreness or fatigue. Serious complications are rare when therapy is delivered in a licensed and controlled medical environment like Regenamex.
Recovery, Results, and Realistic Expectations
Stem cell injection for back pain typically involves minimal downtime, allowing most patients to resume light activities within a few days. Improvements often occur gradually over weeks to months as regenerative signaling influences inflammation, nerve sensitivity, and tissue health.
Potential benefits may include reduced back pain, improved mobility, decreased nerve irritation, and enhanced overall quality of life. Results vary depending on condition severity, lifestyle factors, and adherence to post-treatment recommendations. Regenamex emphasizes realistic expectations, long-term spine care, and patient education throughout the recovery process.
Why Patients Choose Regenamex
Patients from around the world choose Regenamex for stem cell injection for back conditions because of its commitment to safety, transparency, and advanced regenerative medicine. As a COFEPRIS-licensed clinic, Regenamex exclusively uses placental and Wharton’s jelly MSCs and follows strict medical protocols.
With transparent pricing, the Fly & Buy program, personalized treatment plans, and comprehensive aftercare, Regenamex offers international patients access to cutting-edge regenerative care at a fraction of U.S. and Canadian costs—without compromising quality or clinical standards.
Frequently Asked Questions: Stem Cell Injection for Back
Stem cell injection for back pain works by addressing the biological processes that contribute to chronic spinal pain rather than simply suppressing symptoms. Mesenchymal stem cells release anti-inflammatory signals and regenerative factors that help calm ongoing inflammation, support healthier tissue function, and improve cellular communication within discs, joints, and surrounding structures. This approach focuses on improving the long-term environment of the spine instead of providing short-term relief.
Stem cell therapy for back pain is not intended to replace surgery in all cases, but it may help delay or avoid surgical intervention for select patients. Individuals with degenerative disc disease, chronic inflammation, or early structural changes often explore regenerative therapy as an option before committing to invasive procedures. By reducing inflammation and supporting tissue health, stem cell injection for back pain may improve function and decrease symptoms enough to postpone surgery.
Mesenchymal stem cells have demonstrated a strong safety profile in regenerative medicine when properly sourced, processed, and administered. These cells are considered immune-privileged, meaning they rarely trigger rejection or significant immune reactions. This characteristic makes them particularly suitable for spine treatment, where inflammation already plays a major role in symptom development.
The duration of results from stem cell injection for back pain varies depending on factors such as the severity of degeneration, lifestyle habits, overall health, and adherence to post-treatment recommendations. Many patients experience benefits lasting one to two years or longer, particularly when regenerative therapy is combined with proper spine care and physical activity.
Regenerative treatment for spine conditions in Mexico is regulated by COFEPRIS, the federal health authority responsible for overseeing advanced medical therapies. Clinics offering stem cell injection for back pain must comply with strict guidelines related to ethical sourcing, laboratory processing, sterility, and physician oversight.
Most patients undergoing stem cell injection for back pain receive one primary treatment designed to deliver therapeutic benefit over time. The regenerative signaling initiated by mesenchymal stem cells continues to influence the spinal environment for months after administration, which is why multiple sessions are not always necessary.

